74. Preparation of Verdigris for Emerald
Clean a well-made sheet of Cyprian copper by means of pumice stone and water, dry, and smear it very lightly with a very little oil. Spread it out and tie a cord around it. Then hang it in cask with sharp vinegar so that it does not touch the vinegar, and carefully close the cask so that no evaporation takes place. Now if you put it in in the morning, then scrape off the verdigris carefully in the evening, but if you put it in in the evening, then scrape it off in the morning, and suspend it again until the sheet becomes used up. However, as often as you scrape it off again, smear the sheet with oil as explained previously. The vinegar is (thus rendered) unfit for use.
– The Stockholm Papyrus, translated by E. R. Caley
The Stockholm Papyrus is a 4th Century text containing 154 recipes. There are a handful of alloy recipes, and the rest are for lapidary and dyeing. One of the recipes suggests that simulant emeralds can be made with verdigris, and the above quote provides instructions for making it. I’ve started to experiment with this method, partly to test a Graeco-Egyptian recipe, and also teach myself a little chemistry.
Verdigris is a useful material. Simulant stones are mentioned here, but there are several uses for verdigris, including pigments and chrysocolla. The latter means “gold solder”, and can sometimes mean a metal alloy with a lower melting temperature, but in this case refers to eutectic/diffusion/colloidal soldering (pick your preferred name, no-one agrees), where a copper-bearing material reacts with high-carat gold to fuse the components, instead of flooding them with molten metal solder alloys. This was used extensively through history, and is still used by some traditional precious metalworkers. It’s this use that interests me most of all, and as we shall see, it’s not hard to make enough verdigris for that purpose.
This is one of the simplest recipes in the text. The feedstocks are copper, vinegar, and oil. The reaction takes place in a sealed container. The copper is first abraded using pumice, and washed with water. This presents a clean copper surface, free from oxides. “Sharp vinegar” suggests plenty of acetic acid, and I was unsure if household vinegar would be strong enough. The only confusion is the oil – there are many types, and they typically inhibit corrosion in the short term.
This must be a quick procedure; the author suggests scraping the copper twice a day, noting that the sheet will be “used up” by the process, and that the vinegar is rendered “unfit”. A rigid sheet of copper, from maybe 0.3mm upwards, would be the easiest thing to scrape, but a larger surface area could be achieved by hammering it into foil with a thickness below 0.3mm, increasing the yield at the expense of easy scraping.
The container is a cask, which can be “carefully closed…so that no evaporation takes place”. In modern parlance, a cask would be a wooden barrel, but this might well be a ceramic container, or some other material (a glazed pot with a tight lid would be ideal for future experiments). Some method is needed to suspend the copper sheet, so that it can be removed for scraping, and to stop it touching the vinegar.
My eventual aim is to reproduce the recipe, or a functioning variation of it. In the short term, the methodology is simpler, using modern feedstocks, but will hopefully prepare me for an accurate reproduction of the process. Thin copper sheet (0.2mm) is cut into strips, and these are glued with epoxy into the lids of type 1 plastic bottles (100ml), to allow easy removal of the strips. The copper is split into two batches – the first is chemically cleaned, and the second oxidised to a red colour, which should be mostly copper(I) oxide. A strip from each batch is used for all experiments. There are two control samples – one with air, and one with air and distilled water. Unsurprisingly, very little happens in those bottles, at least on the timescale of the recipe.
The overall experiment will cover other reactions, not just verdigris, so 1 strip from each batch is placed into bottles of vinegar, salt, and urine. The other chemicals will be discussed elsewhere; we’ll focus on the verdigris for now. Each bottle is filled to half way up with vinegar, and the copper strip is the same length as the bottle, so that 50% of the copper is submerged, and 50% is exposed to the atmosphere inside the bottle. This is intentionally different to the Stockholm recipe, and likewise, oil has not been used to coat the copper, as the wrong choice of oil will interfere with the reaction.
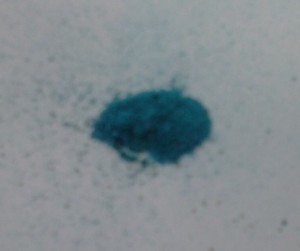
The results are surprisingly good. Within 24hrs, blue-green material had started to appear above the water line. while the submerged copper remained clean. The oxidised copper reacted slightly differently to the clean copper surface, but both showed signs of a blue/green colour. Over a week, a granular, crumbly material had appeared (courser and darker on the oxidised copper sample), and after a couple of weeks, when I got round to scraping them, this turned out to be a plentiful. I’ve wasn’t seeking maximum efficiency here; more regular scraping would present more copper to the vapours, and the delicate strips are inefficient for scraping. In any case, we’re talking about grammes, not kilos, but a small workshop could provide for itself, and scaling up the process would simply require more containers (eg. if using it to produce pigments).
The lower halves, which were submerged in my experiment, are fairly clean – clearly some reactions have taken place, but there is no sign of verdigris. The vinegar has changed colour, gaining a green tinge, suggesting that it should be replaced. Could verdigris be extracted from the liquid? Future experiments will not have copper in contact with the vinegar – it’s a waste of surface area, and the vinegar is suffering for it.
So what are we looking at here? The likely candidate is copper(II) acetate. Vinegar contains acetic acid, which is a weak acid (with only one hydrogen atom to exchange for copper), and will react with a base to form a salt. The base is perhaps a copper oxide, and the resulting salt is copper(II) acetate. If that is the case (it’s likely more complex than that, and my chemistry is rudimentary), then this is a type of verdigris, which can refer to a range of copper compounds, often mixed together, including copper carbonate and copper chloride.
The next step is simple, but more expensive – a better container, more copper, and perhaps a more authentic vinegar. The challenging question is what to do about the “oil” from the recipe. I can experiment with a few plant oils – it might be that they help with the scraping of the plates, rather than the reaction (if the text is inaccurate, it’s possible that the oil is added during the scraping process). Another possibility is that some sort of rancid grease could be used – some of the samples show fingerprints where I’ve touched them, transferring fats and salts onto the surface of the metal. Animal products might promote the formation of a base for the acetic acid to react with.
The next post in this series will deal with reactions with copper and sodium chloride (AKA table salt), which has created a pale green material. I’m also planning to expand the range of samples to include zinc and lead. I want to keep things simple, avoiding multi-stage processes, but I’d like to see the range of materials available to the ancient artisan, using primitive technology.




{ 3 comments }
Hello Jamie,
Very interesting post, I have always admired Ganoksin, and thanked myself for being a part of it, as I have learnt a lot of new techniques and methods which I could not have being so distant from the happening places.
Please keep up the good work of spreading knowledge.
Regards,
Khushroo
I have tried makink “faux” emerals but have little sucess. I will be watching with gtear interest how you do it.
On a side note, I made verdigris by taking a bunch of copper wire and coiling it in a 500 ml beaker. I poured about 2/3rd of a cup of white vinegar in the bottom of a 1000ml beaker and set the small beaker with the copper in the vinegar. Put on the top and let it sit a month. I pulled out the wire and used a large morter and pestle to knock off the verdigris. I ended up with about 2 tablespoons. I just put the container near a vent so it could be warm without making condensation.
When done I just put the copper in the vinegar again. It has been sitting for a good half year now. It is the most intense color. And my vinegar is still clear and working well.
What do you use the verdigris for, Gerald?
Comments on this entry are closed.